Water Purification
An adequate supply of water is absolutely essential to the micro-community life. In addition, the water that will be provided is likely to be infected by microorganism capable to cause various diseases. Therefore we must find and develop an easy, sustainable and cheap technique to purify a reasonable amount of water, at least for drinking and cooking purposes.
A popular way of reliably and cheaply purify the water is the use of halogen tabs. In fact, when dissolved in water, chlorine ions are generated. Those ions are strong oxidizing agents of high toxicity that kill bacteria on contact. However, we cannot consider this solution to be sustainable and realistic, as it will need a constant resupply of halogen tabs to the villages that are in remote and areas difficult of access.
Nevertheless, purification by electrolysis, for its part, is an ambitious solution that is also sustainable and in perfect coherence with our project. Similarly to halogen tabs, if a container of water is subject to electrolysis, hydroxyl and hydronium ions are produced. Then, moving through the liquid from one electrode to another, those ions (especially the Hydroxyl ion) will chemically react with the bacteria to eliminate it10.
In the studies Purification of Floodwater by Electrolysis11and purification of wastewater by electrolysis12, considerable reductions in indicator organisms were obtained through the treatment of floodwater by electrolysis. Based on the results and conclusions of the two studies, we will end up with a technique for purifying water by electrolysis.
Theory:
Electrolysis of water is the decomposition of water molecule (H2O) into oxygen (O2) (will appear at the anode) and hydrogen (H2) ions (at the cathode), due to an electric current being passed through the water. A power source is then connected to the two electrodes, or two plates, which are placed at the two extremity of the water container and thus from which the current passes.
The diagram below describes the container.
Those ions generated by the electrolysis, especially the hydrogen ions, will then come across the bacteria and kill them. The indicator chosen to evaluate the efficiency of the technique is the microorganism concentration before and after the electrolysis.
From theory to practice:
Due to a lack of time and resources, we were unable to conduct our personal experiments so we based our electrolysis method on the robust and reliable experimental results of the second study. However, further arrangements and experiments will be carried before implementation to better improve the technique.
We have then decided to purify the water as follow: Each X minutes a determined small volume of water will be purified by electrolysis and then will be versed from the “electrolyse” container to a “purified water” tank, from which villagers will take provisions (only to drink or cook with). Note that the water fed into the container is assumed to be previously naturally filtered from big particles. In fact, while the water is traveling from higher altitude to lower with a minimum speed, big particles stay stuck on the pipe sides.
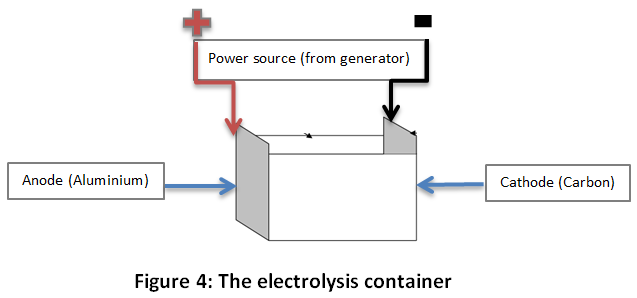
In fact, a 1 Litre “electrolyse” container will be fitted with guide slots on the shorter sides for the electrodes (see figure 4 above). Note, that it is essential to have reliable electrodes that won’t be subject to corrosion. A very smooth 3µm thick and uniform coating on iron sheet electrodes can be considerable as reliable13, but the durability of the electrodes still needs to be determined.
Moreover, regarding the coating of the electrodes, we will prefer having a Carbon cathode and Aluminium anode, as they allow better results. In fact, both studies suggested different treatment of the water before the electrolysis. Adding salt, algae suspension, and especially alum will actually boost the efficiency. However, here we will consider the electrolysis of water without any elements added, as constantly providing those elements is unrealistic and non-sustainable. Nevertheless, we observed that having a Carbon cathode and Aluminium anode (generating Alum ions) will also boost the efficiency as if we were adding Alum14.
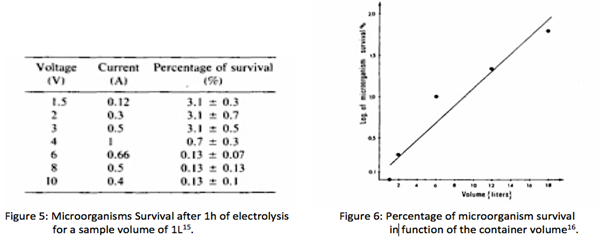
Finally on the figure 5 below, we see that we can eliminate more than 99.87 % of the microorganisms presents in a 1 litre water that contained initially more than 10^6 pathogens/mL. It is done by injecting an AC current of 400 mA at 30 V, during 1hour, which is corresponding to a power consumption of 4.0 W. However, because the efficiency of the purification is inversely proportional to the volume of water (Figure 6), we choose to not purify more than 1L at a time. Therefore, we will design ten 1-litre containers connected in parallel, in order to purify 10 L /hour with an energy consumption of 40 W. h.
However, further experiments will be needed before implementation: we must balance between cost and efficiency, evaluate the durability of the diode and be able to check that the purified water has a microorganism concentration of less than 10^6/mL
[10][11]S. Contrekas, M. Pieber and J. Toha, Purification of Wastewater by Electrolysis, Departmento de Fisica Facultad de Ciencias Fisicas y Matematicas Universidad de Chile Casilla 5487 Santiago de Chile, Chile. December 30, 1980 [12] A. B. M. Badruzzaman and Md. Rezwan Khan, Purification of Floodwater by Electrolysis, Bangladesh University of Engineering and Technology, Dhaka-1000, Bangladesh [13] Purification of Floodwater by Electrolysis, and Purification of Wastewater by Electrolysis . [14] S. Contrekas, M. Pieber and J. Toha, Purification of Wastewater by Electrolysis, Departmento de Fisica Facultad de Ciencias Fisicas y Matematicas Universidad de Chile Casilla 5487 Santiago de Chile, Chile. December 30, 1980 [15][16]S. Contrekas, M. Pieber and J. Toha, Purification of Wastewater by Electrolysis, Departmento de Fisica Facultad de Ciencias Fisicas y Matematicas Universidad de Chile Casilla 5487 Santiago de Chile, Chile. December 30, 1980
In the next section we discuss our distribution management stratagy